
The laws of thermodynamics are a set of principles that govern the behavior of energy and matter in relation to heat and work. These laws are the basis of thermodynamics and apply to a wide variety of physical systems, from heat engines to biological processes and astrophysical phenomena. There are four universally accepted laws, each of which describes essential aspects of the conservation, transfer, and dissipation of energy in systems.
Over time, these principles have been accepted as "laws" because of their universal validity. Although additional formulations have been proposed in recent decades, the four established laws continue to be the fundamental framework of thermodynamics. Interestingly, the zeroth law was formulated after the other three main laws, but because of its fundamental nature, it was assigned the "zeroth" position.
These four laws can be expressed in various ways, depending on the theoretical and practical context in which they are applied. However, their most common formulations are the following:
Zeroth law: Thermodynamic equilibrium
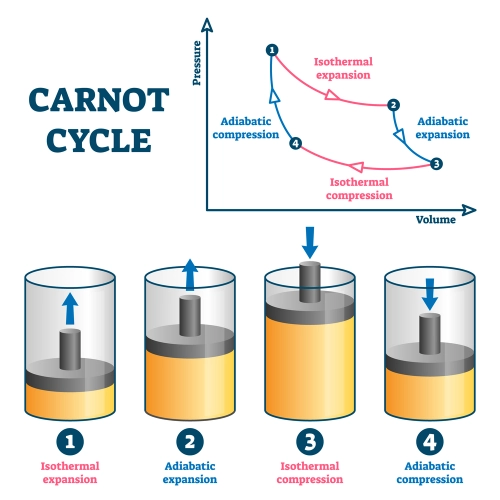 The Zeroth Law states that “if two thermodynamic systems are in thermal equilibrium with a third system, then they are also in equilibrium with each other.” This principle is essential to the definition of temperature and allows for the existence of thermometers and the comparison of temperatures between different bodies. The formulation of this law allowed the rigorous establishment of the concept of thermal equilibrium, a state in which there is no net transfer of heat between the systems in contact.
The Zeroth Law states that “if two thermodynamic systems are in thermal equilibrium with a third system, then they are also in equilibrium with each other.” This principle is essential to the definition of temperature and allows for the existence of thermometers and the comparison of temperatures between different bodies. The formulation of this law allowed the rigorous establishment of the concept of thermal equilibrium, a state in which there is no net transfer of heat between the systems in contact.
In practical terms, this law implies that if an object A is in thermal equilibrium with an object B, and B is in thermal equilibrium with an object C, then A and C are also in thermal equilibrium. This is crucial for the accurate measurement of temperature and for the definition of temperature scales in physics and engineering.
First law of thermodynamics: Conservation of energy
The first law of thermodynamics, also known as the law of conservation of energy, states that "the total energy of an isolated system can neither be created nor destroyed, only transformed from one form to another." This law has broad implications in numerous fields of science and technology, from engineering to biology.
An everyday example of the first law is a heat engine, where the chemical energy of the fuel is converted into thermal energy and subsequently into mechanical work. Mathematically, this law is expressed as:
Where:
- ΔU is the change in internal energy of the system,
- Q is the heat added to the system,
- W is the work done by the system.
This equation reflects that any change in the internal energy of a system is due to heat transfer or work performed. In other words, the energy of a system can only be modified through interactions with its surroundings.
Second law of thermodynamics: Entropy and direction of processes
The second law of thermodynamics states that “the entropy of the universe tends to increase.” Entropy is a measure of the disorder of a system and this law dictates the natural direction of thermodynamic processes. In simple terms, energy is dispersed and systems evolve into states of greater disorder if no external stress is applied.
This law has several important consequences:
- Irreversibility : Some processes, such as the transfer of heat from a hot body to a cold one, are spontaneous and irreversible under natural conditions.
- Efficiency of Thermal Machines : No thermal machine can have 100% efficiency, since there will always be energy losses in the form of heat.
- Evolution of the Universe : The continuing increase in entropy suggests that the universe is evolving toward a state of final thermal equilibrium, known as "heat death."
The mathematical statement of the second law can be expressed by the Clausius inequality:
Where is the amount of heat transferred and is the absolute temperature. In irreversible processes, the total entropy of the system and its surroundings increases.
Third law of thermodynamics: Absolute zero
 The third law of thermodynamics states that “ absolute zero cannot be reached by a finite number of physical processes.” Absolute zero is located at 0 kelvin (-273.15 °C) and represents the lowest possible temperature. At this temperature, theoretically, the particles of a system would be in their fundamental state of minimum energy and the entropy would reach a constant value.
The third law of thermodynamics states that “ absolute zero cannot be reached by a finite number of physical processes.” Absolute zero is located at 0 kelvin (-273.15 °C) and represents the lowest possible temperature. At this temperature, theoretically, the particles of a system would be in their fundamental state of minimum energy and the entropy would reach a constant value.
Mathematically, the third law can be expressed as:
Where:
- S(T) is the entropy at a temperature T ,
- S 0 is the entropy at absolute zero (T = 0) ,
- C p is the specific heat at constant pressure.
- T is the temperature in kelvin.
This law has important implications in materials physics and cryogenics. As a system cools toward absolute zero:
- The entropy of the system is reduced to a minimum value.
- Thermal processes slow down considerably.
- Some substances undergo a phenomenon known as Bose-Einstein condensation, where a large number of atoms occupy the same quantum state.
Although absolute zero is unattainable in practice, understanding this law has enabled advances in superconductivity and the creation of technologies that operate at extremely low temperatures.