
Thermodynamics studies energy changes in physical systems and how these changes affect the properties of matter.
Next, we will show 4 examples that show how thermodynamic processes manifest themselves in our daily lives: internal combustion engines, natural systems, solar thermal energy plants and aerothermal air conditioning.
Internal combustion engines
Internal combustion engines, such as those found in automobiles and motorcycles, are excellent examples of the application of thermodynamic principles. These engines operate in cycles , the most common being the Otto cycle and the Diesel cycle.
Otto cycle
 The Otto cycle is what gasoline engines work through. The ignition of the fuel occurs using a spark and is divided into 4 stages.
The Otto cycle is what gasoline engines work through. The ignition of the fuel occurs using a spark and is divided into 4 stages.
First there is the intake phase, in which the piston moves downward, allowing a mixture of air and fuel to enter the cylinder. This process can be considered approximately isobaric.
Next occurs the compression phase, in which the engine piston moves upward, compressing the mixture. This is an adiabatic process, where pressure and temperature increase.
Subsequently, the mixture is ignited in the combustion phase, generating an explosion that forces the piston downwards. In this stage, the pressure initially increases, and then remains constant in an isobaric process.
Finally, the piston expels the burned gases in the so-called exhaust stage. This expulsion is also carried out in an approximately isobaric process.
Diesel Cycle
The Diesel cycle differs mainly in that the ignition of the fuel occurs by compression, which implies some differences in the thermodynamic processes involved. In this case, it also consists of 4 stages:
In the first stage, air enters the cylinder in the intake stage, in an isobaric process.
Next, in the compression phase, the air is compressed adiabatically, increasing its temperature. Combustion then occurs: the fuel is injected into the hot air, burning and maintaining constant pressure in an isobaric process.
Finally, the burned gases are expelled, in another isobaric process during the exhaust stage.
Natural systems
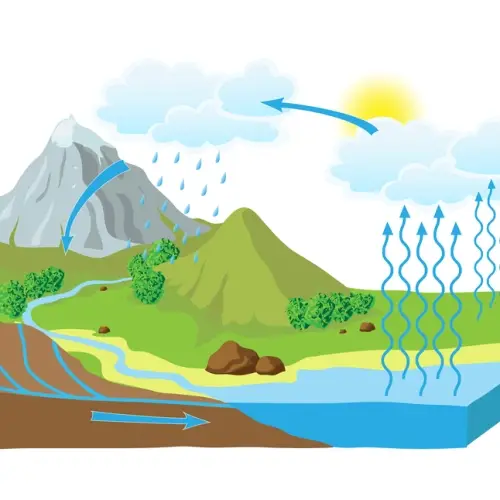 There are numerous natural phenomena that occur due to thermodynamic processes.
There are numerous natural phenomena that occur due to thermodynamic processes.
A prominent example is the water cycle, which involves processes such as evaporation, condensation, and precipitation.
- Evaporation: Water from oceans, rivers and lakes evaporates due to the heat of the sun. This process can be considered an isochoric (constant volume) process because water changes from liquid to vapor state without changing its volume significantly.
- Condensation: Water vapor in the atmosphere cools and condenses into water droplets, forming clouds. This process releases heat, being an exothermic process.
- Precipitation: Water condensed in clouds falls to the ground as rain, snow, or hail. The potential energy of water in clouds is converted into kinetic energy as it falls.
Solar thermal power plants
Solar thermal power plants use the sun's energy to generate electricity. This process involves several thermodynamic steps:
- Heat absorption: Solar collectors capture solar radiation and convert it into heat. This heat is used to heat a working fluid, often thermal oil or water.
- Heat transfer: The hot fluid transfers its energy to a steam generator. In this process, the hot fluid gives off heat to the water, transforming it into steam.
- Electricity generation: High-pressure steam drives a turbine connected to an electrical generator. The expansion of steam in the turbine is an adiabatic process that converts thermal energy into mechanical energy and then into electrical energy.
- Condensation: The steam is condensed back into water and recirculated to the system, completing the cycle.
Air conditioning with aerothermal energy
Aerothermal energy is an efficient technology for air conditioning buildings, using the thermal energy of the outside air to heat or cool interior spaces.
Aerothermal systems are perfect examples for our purpose since they work based on thermodynamic principles.
- Heat Extraction: In heating mode, the heat pump extracts thermal energy from the outside air, even at low temperatures. This process involves the evaporation of a refrigerant within the system, a process that absorbs heat from the environment.
- Compression: The gaseous refrigerant is compressed, raising its temperature and pressure in an adiabatic process.
- Condensation: Hot refrigerant condenses within a heat exchanger, transferring thermal energy to the building's heating system through an exothermic process.
- Expansion: The refrigerant passes through an expansion valve, reducing its pressure and temperature before starting the cycle again.