Thermodynamics, a fundamental branch of physics, unravels the mysteries of heat transfer, and in this vast field, Fourier's law emerges as a cornerstone. This principle, conceived by the illustrious French mathematician and physicist Joseph Fourier in the 19th century, stands as an essential theoretical pillar to understand how heat spreads through materials.
This article seeks to delve into the nuances of this law, challenging the inherent complexity with an accessible and insightful approach. From the mathematical basis to its practical application in everyday situations, we will explore how Fourier's law appears not only in academia, but also in daily life with significant implications.
Who was Fourier?
First, let's take a brief trip back in time to meet the genius behind this law.
Joseph Fourier (1768-1830) was a French mathematician and physicist whose contributions left a lasting mark on science. Born in Auxerre, his achievements include the development of the Fourier series, an essential mathematical tool in the analysis of periodic functions. Fourier also played a crucial role in the theory of heat, proposing the famous Fourier's law, which describes the conduction of heat in materials.
His work allowed him to become a distinguished member of the French Academy of Sciences and left a lasting legacy in the study of differential equations and heat transfer.
Simple explanation of the law
Imagine you are holding a hot cup of coffee. Have you ever wondered how heat travels from coffee to your hands? This is where this thermodynamic law comes into play.
Fourier's law states that the rate of heat transfer through a material is proportional to the temperature gradient. But what is the temperature gradient? It seems complicated, but in reality, it is quite simple. The temperature gradient is simply the temperature difference between two points.
So if there is a large temperature difference between the hot coffee and your hands, the heat will move faster. This makes sense, right? The hotter something is and the colder its surroundings, the faster heat will transfer.
Fourier's law equation
Now, let's talk about the equation that represents Fourier's law in a more mathematical sense. Don't be scared, it's easier than it seems! The equation is:
Let's break it down!
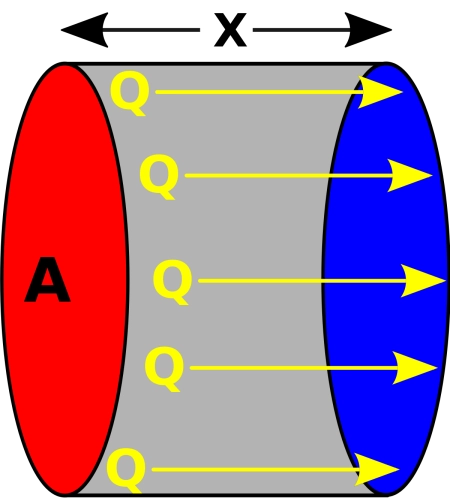
- Q represents the heat transfer rate. It is simply the amount of heat that moves through the material.
- − k is the thermal conductivity of the material. Each material has a unique ability to conduct heat.
- A is the area through which heat is transferred. In the case of the coffee cup, it would be the surface that your hands touch.
- dx/dT is the temperature gradient. This is where the temperature difference we mentioned before comes into play. If you have a large temperature difference in a short space, heat will transfer quickly.
A real example
Now, let's apply Fourier's law to an everyday situation. Imagine that you are cooking a delicious soup in a metal pot. The bottom of the pot is in contact with the hot stove, and you want to know how much heat is transferred to the soup.
First, you need the thermal conductivity of the metal (represented by k ), which is a specific property of each material. Next, you must measure the contact area between the base of the pot and the stove ( A ). Finally, you observe the temperature difference between the hot base of the pot and the soup ( dx/dT ).
Using Fourier's law, you can calculate the amount of heat transferred from the stove to the soup.
Use and applications in real-life
Fourier's Law is not only fascinating from an academic point of view, but it also has practical applications in everyday life. From the design of buildings and their insulation to the manufacturing of electronic devices, understanding how heat is transferred is crucial.
Let's imagine you are designing an energy-efficient house. Knowing the thermal conductivity of the materials used in walls and windows will allow you to create a home that retains heat in the winter and stays cool in the summer.
Example in a solar thermal energy installation
In the field of solar thermal energy, Fourier's law plays a fundamental role, since it is responsible for understanding how heat is transferred through materials, an essential consideration in systems that take advantage of solar radiation to generate thermal energy.
In this section we will analyze the connection between this law of thermodynamics and a solar thermal energy installation:
Capture of solar energy
In a solar thermal energy installation, solar collectors are used to capture solar radiation. These collectors are generally composed of specific materials with well-defined thermal conduction properties.
Fourier's law is applied here by determining how these materials conduct heat from the collector surface to the circulating thermal fluid.
Heat transfer in the thermal fluid
The captured solar radiation is converted into heat, raising the temperature of the thermal fluid (such as water or a specialized thermal fluid) that circulates through the solar collectors.
Our law comes into play when analyzing how this heat is transferred throughout the thermal fluid. The equation of the law, considering the thermal conductivity of the fluid, the transfer area and the temperature gradient, helps to understand and optimize this heat transfer process.
Thermal storage
In many solar thermal energy systems, thermal storage devices are integrated to accumulate the heat generated during periods of maximum solar radiation.
Fourier's law is relevant here when examining how thermal storage materials manage heat transfer during charging and discharging of the system, thus allowing efficient utilization of the stored energy.
Heat transfer to the load
Finally, in a solar thermal installation, the stored heat is used to meet heating demands or to produce steam that can drive turbines and generate electricity.
In this process, the Fourier equation is again applied when considering how heat is transferred from the storage system to the load, whether it is a home heating system or an electrical generation cycle.


